Learn about atoms—the building blocks of the universe— with fun illustrations and examples that are easy for kids to understand and remember.
Get the full 12-page Chemistry PDF for $2.99 here
This colorful 12-page PDF include lessons on the parts of an atom (with a “build an atom” activity), what are quarks and leptons, what are molecules, and meeting the first 12 elements of the periodic table with corresponding flashcards!
or
Download the FREE 3-page Atom PDF by filling in the form below.
Atoms are the building blocks of everything we see! Atoms are so tiny that until a few years ago no one had actually ever seen them, even with a microscope! Atoms are so tiny that light cannot touch them, so they had to invent a different kind of microscope using parts of an atom to even see them! Your hair is 500,000 times wider than an atom. That is really small!
A Greek scientist came up with the name for the atom, the word “Atomos.” This word means “that which cannot be split.”
Imagine a tower of legos. The more you took apart the legos, the smaller the tower would become, until you only had one lego left. If you broke apart the lego, it would no longer be a lego, but only plastic bits. Atoms are like legos, the building blocks of matter. Like legos, atoms can combine with other atoms to form new things, but all ordinary matter is made of atoms. These atoms combine to form everything we see and touch— even the air!
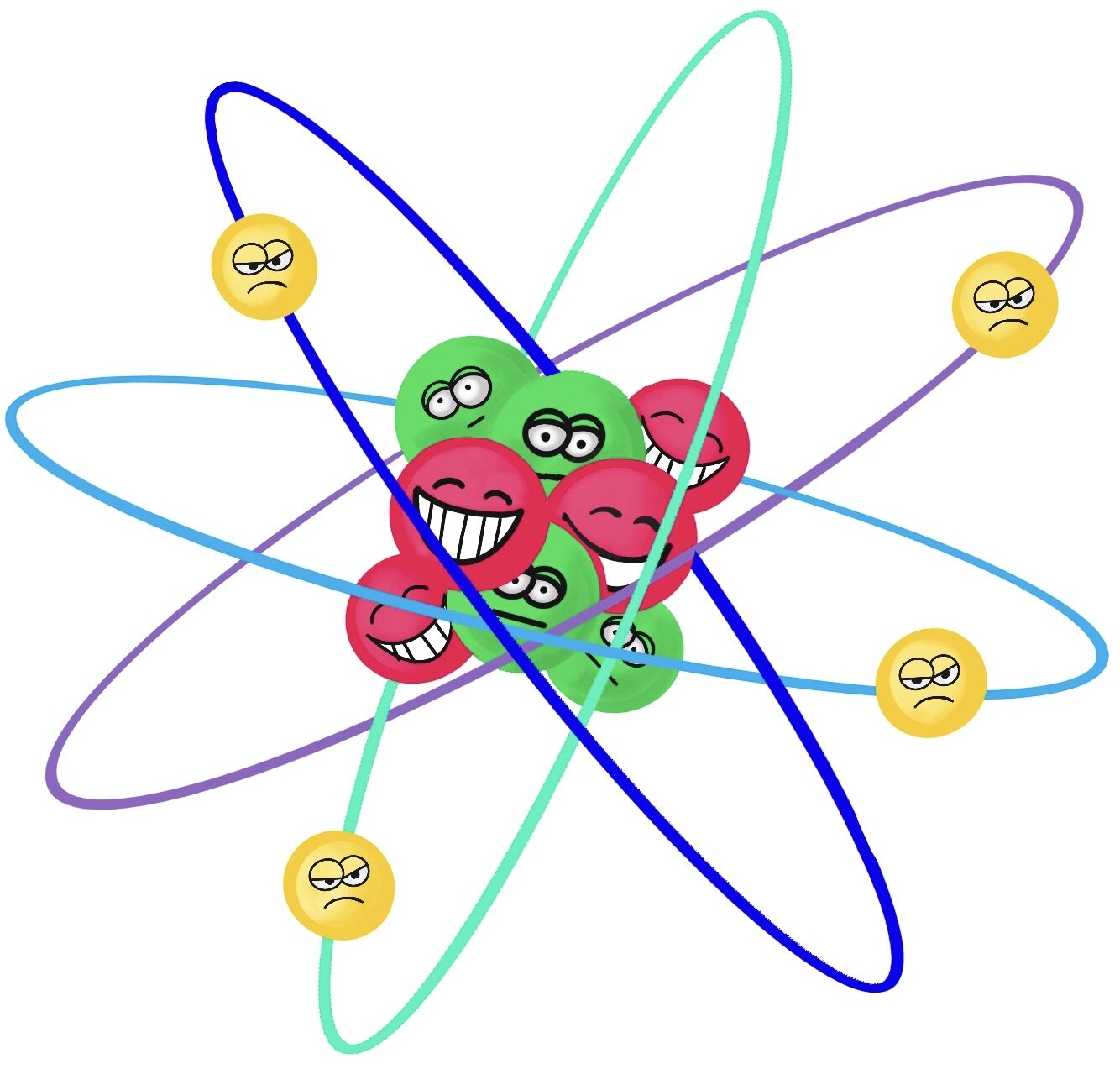
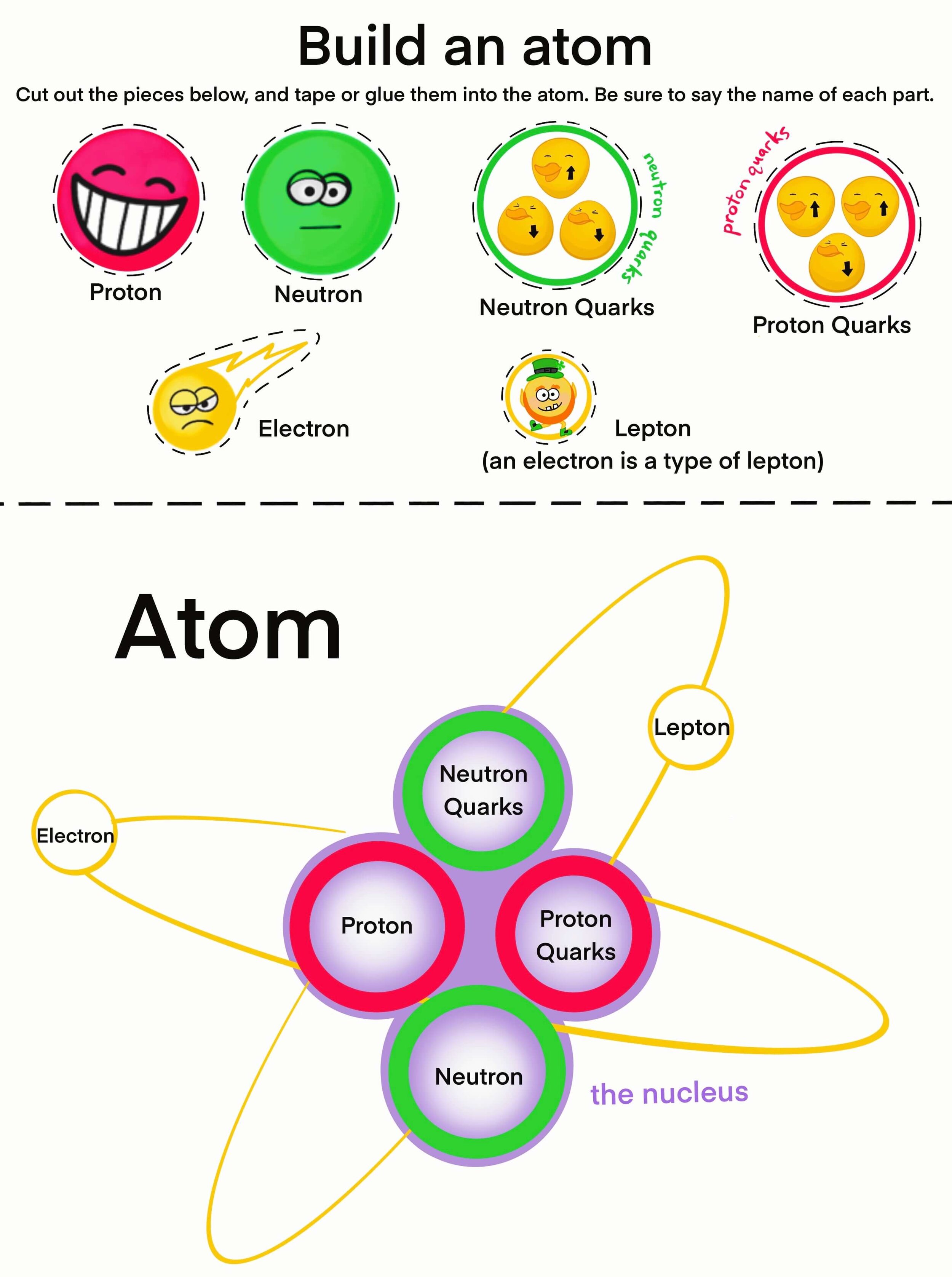
Parts of an Atom
The basic parts of atoms are protons, neutrons, and electrons. The protons and neutrons form a tight ball in the center called a nucleus. The electrons zoom around the outside of the nucleus.
The nucleus is made of protons and neutrons. Protons are positive- see how they smile? They give atomʻs their identity. If the atom has 2 protons, it is helium. If the atom has 47 protons, it is silver. The atom can have a different number of neutrons or electrons, but the number of protons it has tells us what element the atom is.
Neutrons help protons stick together in the nucleus.
Neutrons act like a glue to keep the protons together. Opposites attract, and like charges repel, so if the positively charged protons were all pushed together, they would repel each other. The neutrons are neutral, meaning they have no charge, so they keep all the positive protons from repelling each other.
Electrons have a negative charge— see how negative they are with their grumpy faces? Electrons orbit the nucleus super fast. They are so fast that the electrons forms what seems like a shield around the outside of the nucleus.
Electrons spin and rotate around the outside of the nucleus— kind of like planets orbiting the sun! As the electrons circle the nucleus, they travel at certain energy levels but can "jump" between different energy levels if they gain or lose energy.
Letʻs remember these names with some gestures:
Protons (smile and thumbs up!)
Neutrons (arms at your sides)
Nucleus (clasp your hands together),
Electrons (point your fingers and spin them in a fast circle in front of you.)
Zap-Pow! Try it!
Rub your feet on carpet.
You can make charged atoms. As you rub your feet on carpet, you pick up extra electrons from the carpet. This causes static electricity. If you reach for a friend after rubbing your feet on the carpet, you might feel a little zap. This zap is the electrons traveling from you to another place.
Rub a balloon on your head. When you rub a balloon on your head, you rub electrons off your head onto the balloon. The atoms missing electrons are positive, and the extra electrons are negative. These atoms have opposite charges, so they are attracted to each other. This will make your hair attracted to the balloon!
Click here to get the 12-page Chemistry PDF! Includes lessons on atoms, quarks, leptons, molecules, flashcards of the first 12 elements of the periodic table.